Quantitative Assessment of Wheat Pollen Shed by Digital Image Analysis of Trapped Airborne Pollen Grains
Received: 16-Nov-2013 / Accepted Date: 17-Dec-2013 / Published Date: 19-Dec-2013 DOI: 10.4172/2329-8863.1000119
Abstract
The objective of the present study was to develop a technique for quantifying the dynamics of wheat pollen shed under field conditions. Pollen traps with an adhesive film were used to assess the relative pollen shed of 12 winter wheat lines. Quantitative measurements were performed in 2012 and 2013 over a period of up to 20 days. The amounts of trapped pollen were automatically determined using a customized image analysis program. We demonstrated that this method is suitable for the assessment of wheat pollen shed. The possible impact of the technical advances revealed in this study for the selection of pollinators in routine wheat breeding programs is discussed.
Keywords: Pollen shed, Pollen traps, Digital image analysis, Field trials, Hybrid wheat
Introduction
Wheat (Triticum aestivum L.) is predominantly a self-pollinating crop. Still, outcrossing via pollen dispersal is possible at variable rates [1]. The rate of outcrossing depends upon genotypes, populations and environmental conditions and directly correlates to the amount of wind-borne pollen [2-4].
In the vast majority of studies, the outcrossing capability of wheat plants was measured by their ability to fertilize crossing partners. Such examinations were conducted either on male-sterile crossing partners [5-8] or under natural conditions of pollen competition [3,9,10].
However, in order to evaluate the potential of wheat plants for out-crossing, an efficient method for quantifying the dynamics of wheat pollen shed would be highly advantageous. An approach to the measurement of wheat pollen shed may include a device suitable for capturing the pollen that is shed over a certain period of time (“Pollen trap”) coupled with a tool for counting of pollen. Pollen traps may be active or passive, depending on whether they actively suck in air containing pollen or whether they rely on the passive transport of pollen by wind. Previously, the use of active pollen traps [11] or passive pollen traps [12,13] was described to measure wheat pollen dispersal under open- field. The pollen number was determined by counting the pollen grains in microscopic images through visual inspection. The use of image analysis software for the quantification of pollen shed was reported for maize [14].
In this technical report, we describe the combined use of a new type of passive adhesive pollen trap with image analysis software that was specially tailored to the needs of wheat pollen analysis. We assume that this method offers the potential to facilitate a reliable quantitative assessment of wheat pollen shed and that this technology may be utilized for the identification of wheat lines with high pollen shed. Since high amounts of air-borne pollen are a prerequisite for successful commercial hybrid wheat production, we believe that the described method has a direct value for wheat breeding programs.
Materials and Methods
Field trials were conducted in 2012 and 2013 at the breeding station of Nordsaat Saatzucht GmbH located in Langenstein, Germany (51°53’N, 10°59’E). A total of 12 winter wheat lines were analyzed. All of the lines were bred in identical field plots of 7 by 9 m at a plant density of 300/m2.
Pollen capture
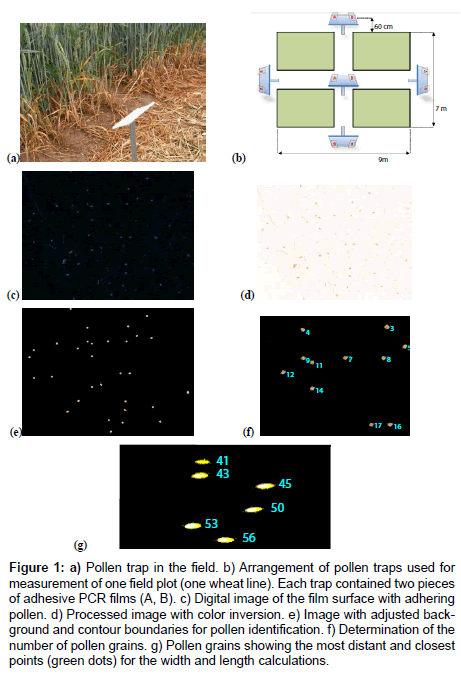
For pollen capture, two slides of optically clear adhesive seal sheets for polymerase chain reaction (PCR) multiwell plates (AB-1170, Thermo Fisher Scientific, Schwerte, Germany) were fixed with clamps on a metal base (14 by 10 cm) and mounted at a 45° vertical angle and 40 cm height on a metal pole (Figure 1a). In the experiments carried out in 2012, the pollen traps were placed at each end and in the center of each field plot as depicted in Figure 1b. The distance to the pollen source was 60 cm. The slides were exchanged twice daily at 7:00-8:00 a.m. and 5:00-6:00 p.m. In the experiments that were conducted in 2013, the number of traps was reduced from five to three and pollen capture was carried out only over the day (7:00 a.m. to 6:00 p.m.) while the remaining experimental parameters were identical to the field trials of the previous year. We have chosen these periods of time on the basis of a case study in which the maximum release of pollen was found to occur from 8 to 11 a.m. and a less pronounced peak was found at 3 to 6 p.m [15].
Figure 1: a) Pollen trap in the field. b) Arrangement of pollen traps used for measurement of one field plot (one wheat line). Each trap contained two pieces of adhesive PCR films (A, B). c) Digital image of the film surface with adhering pollen. d) Processed image with color inversion. e) Image with adjusted background and contour boundaries for pollen identification. f) Determination of the number of pollen grains. g) Pollen grains showing the most distant and closest points (green dots) for the width and length calculations.
Sealing/conservation of traps
Immediately after collection, the adhesive films with captured pollen were covered with a transparent sheet to facilitate sealing of the pollen trap for archiving and subsequent analysis.
Selection of adhesive surfaces for pollen capture
Pilot experiments were conducted in June 2011. Different adhesive films were tested for their ability to collect wheat pollen under field conditions and then assessed for their suitability for use in microscopic analyses. Due their easy handling and high quality standards (homogenous dispersion of the adhesive, stiffness, transparency) adhesive PCR seal foils were applied. The particular films tested were E2796-9793 and E2796-9795 from Starlab GmbH (Hamburg, Germany), AB-1170 and AB-0558 from Thermo Fisher Scientific (Schwerte, Germany) and UC-500 from Axygen (Union City, CA, USA). Four samples of each film were fixed on metal plates and arranged around a wheat field (pollen source). The films were exposed from 8 to 11 a.m. and then sealed for microscopic analysis. All the films had a comparable capability to collect pollen, but we observed variation in their suitability for microscopic analysis.
In general, the thinner seal films used for real-time PCR applications (E2796-9795, AB-1170 and UC-500) were more suited for microscopic examination because of their higher transparency and more homogenous texture, which results in a higher accuracy of the automated pollen assessment. In comparison to the real-time PCR seals, use of E2796-9793 and AB-0558 led to an error rate of 25-30% when pollen was counted automatically (i.e. 25-30% of the pollen that was detected by visual detection was not identified by the software EVALUATOR). In case of the real-time PCR seals, an average deviation of ± 2% between automatically counting and visual inspection was measured.
In addition, all of the seals were tested for their performance at rain. For this purpose, three of each seal were exposed to a flowering wheat field from 7 a.m. to 6 p.m. at a rainy day. Whereas the adhesives of the seals E2796-9793 and AB-0558 were negatively affected by humidity (tendency to cloudiness and in-transparency) the real-time PCR seals could be dried easily during the sealing without adverse effects. Among the real-time PCR foils, AB-1170 was chosen for further analysis because of its favorable price and availability in higher quantities.
To evaluate whether any unequal distribution of pollen occurs on the surface, up to 10 images were analyzed per trap. We did not find any obvious bias in pollen deposition on the trap surface at any pollen shed density. The average of the pollen numbers collected on two randomly chosen areas of 0.25 cm2 was used as the measured value for each pollen trap.
Digital image analysis
The digital images were produced using a Zeiss AxioCam digital camera system with AxioVision software in combination with a Stemi 2000 microscope (Zeiss, Jena, Germany). The images were captured at 16X magnification (Figure 1c). The camera exposure time was 0.7 s.
The program EVALUATOR was used following the production of the digital image to generate an automated color-inverted image (Figure 1d). Subsequently, the software algorithm isolates the pollen grains from the background based on differences in pixel intensities and creates a contour boundary for the precise identification of each grain and its orientation, along with a new background for the grains with definable RGB values. The pollen grains appear as bright spots (Figure 1e). The customized software determines the number of grains and numbers them consecutively in the digital image (Figure 1f). Furthermore, the two most distant and the two closest points on opposite sides of the grain are identified, and the linear distances between the two sets of points are defined as the length and the width, respectively (Figure 1g). The determination of the pixel number inside the boundaries enables the calculation of the two-dimensional area of the pollen grain.
The particles whose shapes and sizes are not within a selected range are reliably removed from the image. In most cases, touching or misshaped grains are recognized and excluded from the analysis (not counted) because their size is out of a defined range that is set by the operator. All filters and threshold parameters can be manually set by the operator.
As an alternative option, the program offers the opportunity to check questionable cases by visual inspection of the screen picture. Structures can be added or subtracted to the count if appropriate. This approach was used in some cases in which the adhesive surface was scratched or excessively covered with dust.
Notably, the software allows the filter and threshold conditions optimized for one image to be automatically applied to all other images of the same project without further adjustments, which enables highthroughput image analysis. The images (processed and unprocessed) are stored along with the report document in BMP format. The summarized results of the analysis can be exported to an SCV file, and the measured values (including the number of pollen grains, the number of rejected objects and the pollen length, width and area) are stored as an Excel file. The program EVALUATOR works under Windows.
For this study, the Delphi software EVALUATOR was adapted according to the specific requirements for the examination of wheat pollen. Other versions of the EVALUATOR software were recently used to analyze the size of Arabidopsis thaliana cotyledons [16] and seeds (Boudichevskaia and Schmidt, unpublished results).
Results
Field experiments in 2012 (Experiment I)
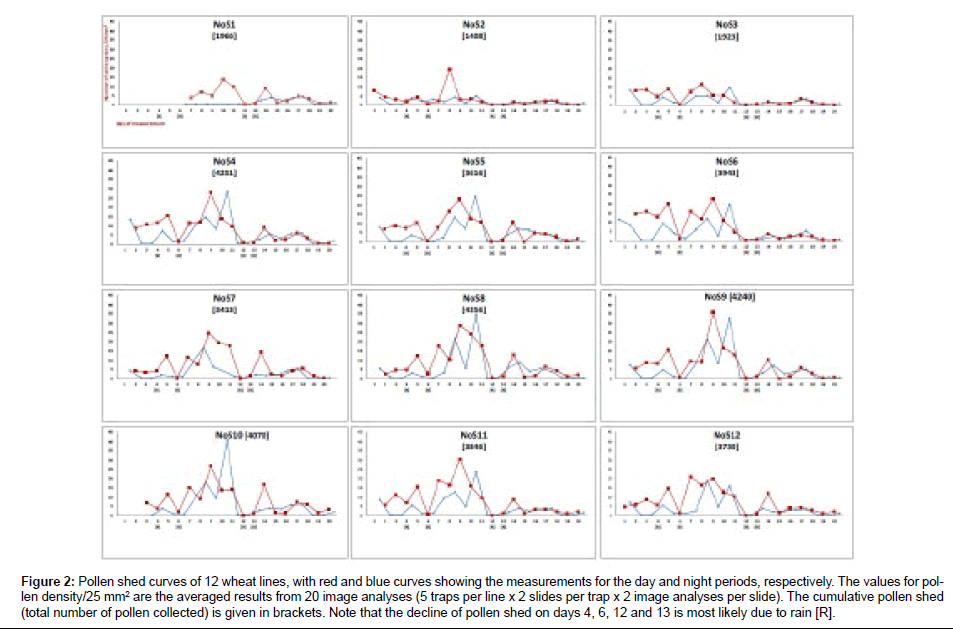
The relative dynamics of pollen shed for 12 winter wheat lines was measured (Figure 2). Measurements were initiated for each line at the individual start of anthesis. The data points for day and night values were expressed as the mean number of pollen grains counted in 20 image analyses (5 traps/line×2 slides per trap×2 images per slide; mean value=total number of counted pollen/20). Most of the wheat lines displayed a similar temporal pattern of pollen shed in which the shedding increased until day 9, when the number of pollen grains reached a maximum of between 21 and 36 per 25 mm2 of trap surface. The lines NoS1, NoS2 and NoS3 exhibited lower pollen shed than the other lines and a later initiation of anthesis. As expected, pollen shed was reduced during the night, with the exception of night 10, when large amounts of pollen were collected. However, this outlier can be attributed to exceptionally strong nocturnal winds.
Figure 2: Pollen shed curves of 12 wheat lines, with red and blue curves showing the measurements for the day and night periods, respectively. The values for pollen density/25 mm2 are the averaged results from 20 image analyses (5 traps per line x 2 slides per trap x 2 image analyses per slide). The cumulative pollen shed (total number of pollen collected) is given in brackets. Note that the decline of pollen shed on days 4, 6, 12 and 13 is most likely due to rain [R].
On several days (4, 6, 12 and 13), it was raining during the period of measurement, and the amount of captured pollen decreased significantly. This decrease can be explained by either the removal of pollen from the trap, a lower adhesive force of the trap, reduced pollen shed by the plants or a combination of these factors.
The image analysis results were confirmed on more than 200 slides by visually counting the pollen using unprocessed images. Overall, a high accordance between the counting methods was found (average deviation of ± 2%). The counting errors appeared predominantly at higher pollen densities when the grains tended to touch each other. Small particles, such as dust, and larger objects such as insects or anthers were reliably removed during image processing. From our results we concluded that that the automated pollen counts were highly accurate.
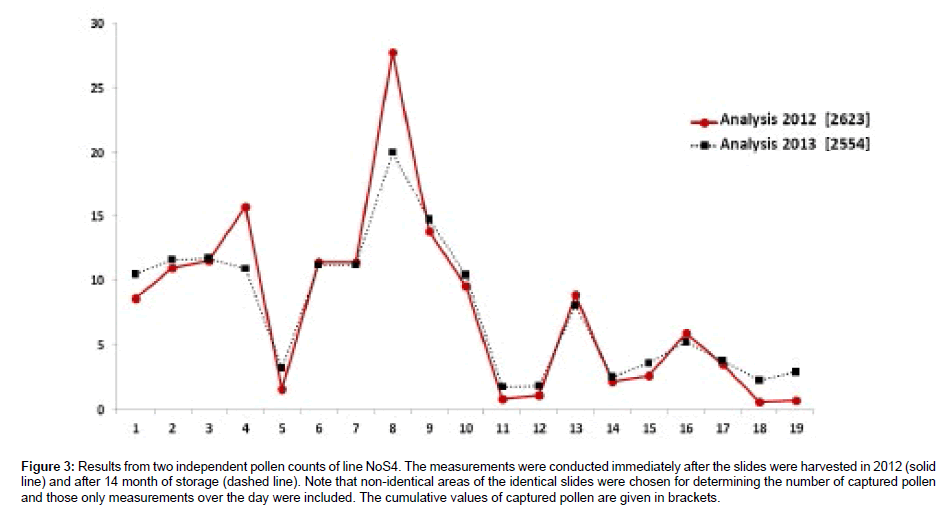
Pollen counting was conducted immediately after harvesting the traps in 2012, and, in a second analysis, after storage of 14 months (Figure 3). The close match between the two sets of data indicates that the traps can be stored for an extended period of time under ambient conditions without significantly affecting the counting results.
Figure 3: Results from two independent pollen counts of line NoS4. The measurements were conducted immediately after the slides were harvested in 2012 (solid line) and after 14 month of storage (dashed line). Note that non-identical areas of the identical slides were chosen for determining the number of captured pollen and those only measurements over the day were included. The cumulative values of captured pollen are given in brackets.
Field experiments in 2013 (Experiment II)
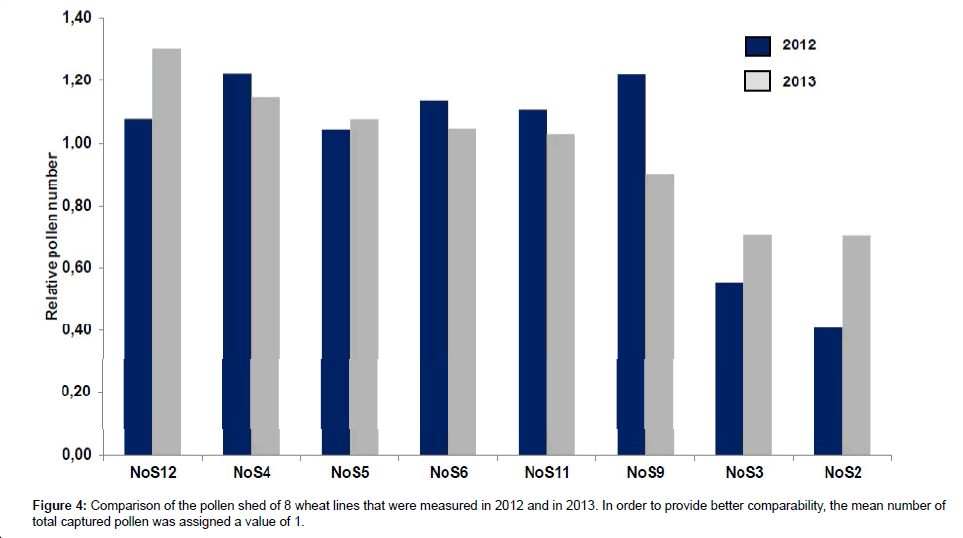
In 2013, the pollen shed measurements of eight of the 12 winter wheat lines were repeated. Lines were chosen that represented both classes of high and low-pollen shedding genotypes. Among the selected lines were two lines that showed low pollen shed in 2012 (lines NoS2 and NoS3; line NoS1 was excluded because of the late flowering phenotype), two lines with the highest cumulative amounts of pollen counted in 2012 (NoS4 and NoS9) and four lines with medium pollen shed in 2012.
Figure 4 compares the relative values of the total pollen count measured for both sets of experiments. The relative values for each particular genotype in the chart are calculated as (total number of pollen captured from the particular genotype per year)/(average pollen number captured from the all genotypes per year). The results obtained from both experiments display a significant correlation (correlation coefficient=0.77; p<0.01). From these data we deduce that the described method is in general a suitable tool for a comparative assessment of winter wheat pollen shed. A certain variation between the pollen shed in 2012 and 2013 is expected since the weather conditions were not identical; however, from our results it is possible to confirm the categorization of the lines into classes with high pollen shed and such lines that display relative low levels of pollen shed (NoS2 and NoS3).
Pollen size
The program EVALUATOR also allows for the measurement of pollen size. In total, 16,000 pollen grains were analyzed. The mean size was 84 μm (± 2.8 μm). An ANOVA revealed that there was no statistically significant difference in pollen size among the different wheat lines investigated (p=0.94). Nevertheless, the results of the measurements have to be interpreted with care. The repositioning of the samples during image production may move the pollen grains out of the optimal optical focus plane which might contribute to differences in the measured object sizes.
Discussion
Techniques for estimating the degree of pollen grain shed are important for many topics related to pollination biology. The role of pollen in pathogenesis of allergic diseases is well established [17]. Also, in recent years, pollen dispersal in wheat has gained increasing attention due to the potential of GM crops to transfer foreign genes from GM plants to related non-GM plants [18,19].
The method described in this article appears to be suitable for characterizing the temporal pattern of wheat pollen shed under field conditions. An important practical application of the technique for plant breeding might be the identification of wheat lines that have the prerequisites to constantly and reliably pollinate females in hybrid production. Previous studies on pollen dispersal focused on the commercial production of hybrid wheat, where reaching high levels of genetic purity and sufficient seed set on male sterile plants were essential [20].
Hybrids often display a yield increase, enhanced yield stability and improved abiotic and biotic stress resistance due to the exploitation of heterosis (hybrid vigor) [21,22]. When applied on a large scale, hybrid wheat is the product of the controlled crossing of two pure lines (a sterile “mother line” and al “male” pollinator) in a propagation field. Despite its outstanding performance, hybrid wheat currently occupies only a niche sector in commercial wheat production. One major reason for this is that the production of “good males” may require significant breeding efforts [20,23] because excellent pollinator characteristics (e.g., amount of pollen shedding, anther extrusion and length, duration of pollination, pollen viability) have to be available in combination with high breeding values. It is estimated that suitable pollinators constitute only 1-2% of the modern wheat varieties (Schachschneider, unpublished results). The reason for this rareness is that a number of characteristics, particularly the pollen and floral biology, render wheat ill-adapted to cross-fertilization [1,5,23,24]. Moreover, compared with other grass species, wheat produces a small amount of pollen, and the pollen settles quickly because it is relatively heavy [25] Thus, wind is required to move wheat pollen over an appreciable distance [2,26]. Furthermore, wheat pollen is viable for a relatively short period of time. In a previous study, its viability was completely lost within 65-70 min [27].
In contemporary hybrid wheat breeding programs, pollinator lines are selected by testing their ability to cross-fertilize male-sterile females in so-called “experimental crossing blocks” [8,28]. This method presents several disadvantages, such as high operation costs, the risk of failure of the chemical hybridization agent and the necessity of using male and female lines that have an overlapping flowering period [29,30]. The technology described in this study appears to be advantageous for the precise selection of pollinator candidates because only the male component is involved. The removal of bottlenecks to progress in the identification of pollinators may lead to considerable cost savings. Nevertheless, in the future, it will be important to determine to what extent the measured differences in pollen shed correlate with different rates of seed set on the female lines.
It is difficult to compare the absolute numbers of captured wheat pollen with those obtained in previous studies because the experiments varied regarding the distance of the traps from the pollen source and the type of trap used. Moreover, the measurements were made at other locations and different wheat lines were analyzed. Yet, the maximum number of pollen grains per area of slide surface was comparable with the values published in a study of wheat [12] and, as expected, significantly lower (~30x) than those reported for efficient pollinators, such as maize [14]. The quantity of captured pollen was considerably reduced by rain, and additional environmental factors and their interactions, such as the wind speed and direction, air turbulence, temperature and humidity, may influence the amount of trapped pollen. Therefore, to further substantiate claims about the pollination capabilities of different wheat lines, future trials should be carried out at multiple locations. Still, since it can be assumed that rainfall efficiently inhibits the overall pollen flow, the low values measured on rainy days may represent a realistic scenario. Nevertheless, future pollen traps might be equipped with devices that protect the slides from rain in order to prevent that already captured pollen is washed off the surface.
The proven storability of the pollen-covered slides might offer a major technical advantage over traps that collect pollen in isotonic solutions [31]. Several morphological flower and plant characteristics may influence the amount of pollen shed [1,23,26]. While observations of several characteristics, for example, anther extrusion or plant height, are readily obtainable, the analysis of others, such as pollen number or size, is more difficult. For breeders, it would be highly interesting to specifically correlate plants characteristics with the dynamics of pollen shed in order to enable a selection of pollinators via “indirect” traits. The availability of an efficient technology for assaying the pollen would strongly facilitate correlation studies. We hope that the describe method will be a valuable tool for such on-field plant phenotyping.
Acknowledgements
The authors are grateful to Iris Röhlich, Karoline Buller, Corinna Schollmeier and Manja Franke for their excellent technical support. We thank the federal ministry of education and research for funding the project “KMU-Innovativ” (funding code 0315889) as a joint project between the IPK Gatersleben and the Nordsaat GmbH (Langenstein, Germany). The work was also supported by a BMBF grant to Renate Schmidt (funding code 0315053G).
References
- Waines JG, Hedge SG (2003) Intraspecific gene flow in bread wheat as affected by reproductive biology and pollination ecology of wheat flowers. Crop Science 43: 451-463.
- Beckie HJ, Hall LM (2008) Simple to complex: Modelling crop pollen-mediated gene flow. Plant Science 175: 615-628.
- Hucl P (1996) Out-crossing rates for 10 Canadian spring wheat cultivars. Can J Plant Sci 76: 423-427.
- Matus-Cadiz MA, Hucl P, Dupuis B (2007) Pollen-mediated gene flow in wheat at a commercial scale. Crop Science. 47: 573-581.
- Arya RK, Sethi SK (2005) Studies on floral traits influencing outcrossing in wheat (Triticum aestivum L.). National Journal of Plant Improvement 7: 73-76.
- de Vries AP (1974) Some aspects of cross-pollination in wheat (Triticum aestivum L.). 4. Seed set on male sterile plants as influenced by distance from the pollen source, pollinator: male sterile ratio and width of the male sterile strip. Euphytica 23: 601-622.
- Lu AZ, Zhao H, Wang TY, Wang HB (2002) Study of possibility of target gene introgression from transgenic wheat into non-transgenic plants through pollens. Acta Agric Bor Sin 17: 1-6.
- Wilson JA, Ross WM (1962) Cross Breeding in Wheat, Triticum aestivum L. II. Hybrid Seed Set on a Cytoplasmic Male-Sterile Winter Wheat Composite Subjected to Cross- Pollination. Crop Science 2: 415-417.
- Hucl P, Matus-Cadiz MA (2001) Isolation distances for minimizing outcrossing in spring wheat. Crop Science 41: 1348-1351.
- Matus-Cadiz MA, Hucl P, Horak MJ, Blomquist LK (2004) Gene flow in wheat at the field scale. Crop Science 44: 718-727.
- Alcázar P, Galán C, Cariñanos P, Domínguez-Vilches E (2003) A new adhesive for airborne pollen sampling in Spain. Aerobiologia 19: 57-61.
- Loureiro I, Concepción Escorial M, González-Andujar JL, García-Baudin JM, Chueca MC (2007) Wheat pollen dispersal under semiarid field conditions: potential outcrossing with Triticum aestivum and Triticum turgidum. Euphytica 156: 25-37.
- Sapra VT, Hughes JL (1975) Pollen production in hexaploid Triticale. Euphytica 24: 237-243.
- Fonseca AE, Westgate ME, Doyle RT (2002) Application of fluorescence microscopy and image analysis for quantifying dynamics of maize pollen shed. Crop Science 42: 2201- 2206.
- de Vries AP (1972) Some aspects of cross-pollination in wheat (Triticum aestivum L.) 1. Pollen concentration in the field as influenced by variety, diurnal pattern, weather conditions and level as compared to the height of the pollen donor. Euphytica 21: 185-203.
- Meyer RC, Witucka-Wall H, Becher M, Blacha A, Boudichevskaia A, et al. (2012) Heterosis manifestation during early Arabidopsis seedling development is characterized by intermediate gene expression and enhanced metabolic activity in the hybrids. Plant J 71: 669-683.
- Sofiev M, Bergmann KC (2013) Allergenic Pollen: A Review of the Production, Release, Distribution and Health Impacts. Springer 247.
- Conner AJ, Glare TR, Nap JP (2003) The release of genetically modified crops into the environment. Part II. Overview of ecological risk assessment. The Plant J 33: 19-46.
- Singh OV, Ghai S, Paul D, Jain RK (2006) Genetically modified crops: success, safety assessment, and public concern. Appl Microbiol Biotechnol 71: 598-607.
- Pickett AA (1993) Hybrid wheat-results and problems. In: Advances in Plant Breeding, Paul Parey Scientific Publishers, Berlin 15.
- Longin CF, Gowda M, Muhleisen J, Ebmeyer E, Kazman E, et al. (2013) Hybrid wheat: quantitative genetic parameters and consequences for the design of breeding programs. Theor Appl Genet 126: 2791-2801.
- Mühleisen J, Piepho HP, Maurer HP, Longin CF, Reif JC (2013) Yield stability of hybrids versus lines in wheat, barley, and triticale. Theor Appl Genet. 127(2): 309-16.
- Whitford R, Fleury D, Reif JC, Garcia M, Okada T, et al (2013) Hybrid breeding in wheat: technologies to improve hybrid wheat seed production. J Exp Bot 64(18): 5411-28
- Gatford KT, Basri Z, Edlington J, Lloyd J, Qureshi JA, et al. (2006) Gene flow from transgenic wheat and barley under field conditions. Euphytica 151: 383-391.
- Lelley J (1966) Observation on the biology of fertilization with regard to seed production in hybrid wheat. Der Züchter 36: 314-317.
- Gustafson DI, Horak MJ, Rempel CB, Metz SG, Gigax DR, et al. (2005) An Empirical Model for Pollen-Mediated Gene Flow in Wheat. Crop Science 45: 1286-1294.
- Fritz SE, Lukaszewski AJ (1989) Pollen longevity in wheat, rye and triticale. Plant Breeding 102: 31-34.
- Porter KB, Lahr KA, Atkins IM (1965) Cross-Pollination of Male-Sterile Winter Wheat (Triticum aestivum L.) Having Aegilops caudata L. and Aegilops ovata L. Cytoplasm. Crop Science 5: 161-163.
- Curtis BC, Rajaram S, Gomez Macpherson H (2002) Bread wheat: improvement and production. FAO Plant Production and Protection Series.
- Kempe K, Gils M (2011) Pollination control technologies for hybrid breeding. Plant Breeding 27: 417-437.
- Carre S, Tasei JN (1997) Use of a flow-cytometer for pollen counting-An application to Vicia faba. L. Acta Hortic 437: 369-372.
Citation: Kempe K, Boudichevskaia A, Jerchel R, Pescianschi D, Schmidt R, et al. (2013) Quantitative Assessment of Wheat Pollen Shed by Digital Image Analysis of Trapped Airborne Pollen Grains. Adv Crop Sci Tech 2:119. Doi: 10.4172/2329-8863.1000119
Copyright: © 2013 Kempe K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 17946
- [From(publication date): 2-2014 - Apr 24, 2024]
- Breakdown by view type
- HTML page views: 13425
- PDF downloads: 4521