Influence of Biofield Treatment on Physicochemical Properties of Hydroxyethyl Cellulose and Hydroxypropyl Cellulose
Received: 29-May-2015 / Accepted Date: 09-Jul-2015 / Published Date: 20-Jul-2015 DOI: 10.4172/2329-9053.1000126
Abstract
Cellulose based polymers have shown tremendous potential as drug delivery carrier for oral drug delivery system (DDS). Hydroxyethyl cellulose (HEC) and hydroxypropyl cellulose (HPC) are widely explored as excipients to improve the solubility of poorly water soluble drugs and to improve self-life of dosage form. This work is an attempt to modulate the physicochemical properties of these cellulose derivatives using biofield treatment. The treated HEC and HPC polymer were characterized by X-ray diffraction (XRD), differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The XRD studies revealed a semi-crystalline nature of both the polymers. Crystallite size was computed using Scherrer’s formula, and treated HEC polymer showed a significant increase in percentage crystallite size (835%) as compared to the control polymer. This higher increase in crystallite size might be associated with greater crystallite indices causing a reduction in amorphous regions in the polymer. However treated HPC polymer showed decrease in crystallite size by -64.05% as compared to control HPC. DSC analysis on HEC polymer revealed the presence of glass transition temperature in control and treated HEC polymer. We observed an increase in glass transition temperature in treated HEC, which might be associated with restricted segmental motion induced by biofield. Nonetheless, HPC has not showed any glass transition. And no change in melting temperature peak was observed in treated HPC (T2) however melting temperature was decreased in T1 as compared to control HPC. TGA analysis established the higher thermal stability of treated HEC and HPC. CHNSO results showed significant increase in percentage oxygen and hydrogen in HEC and HPC polymers as compared to control samples. This confirmed that biofield had induced changes in chemical nature and elemental composition of the treated polymers (HEC and HPC).
Keywords: Hydroxyethyl cellulose; Hydroxypropyl cellulose; XRD; DSC; TGA; Biofield treatment
Introduction
The oral route is by far the most preferred and convenient route for delivery of many pharmaceutically active drugs. Thus, the oral mucosa has many properties that make it a fascinating choice for drug delivery [1]. Oral drug delivery is an excellent non-invasive approach that provides alternative to invasive routes such as intravenous, intramuscular, subcutaneous administration of drugs. Nevertheless, it also provides several challenges for pharmaceutical scientist investigating novel delivery techniques to overcome. There are different formulations strategies including sprays, tablets, mouthwashes, gels, pastes and patches are currently used for delivery into and across the oral mucosa. DDS developed for local delivery to mucosal diseases require different pharmacokinetic behavior compared to topical delivery for systemic applications [1]. Presently, there are a small number of drugs which are routinely delivered via the sublingual or buccal route e.g. systemic delivery of glyceryl trinitrate for angina relief and topical corticosteroid administration for inflammatory diseases of the oral mucosa including lichen planus [2]. Nevertheless, the formulations administered orally face a daunting challenge by acidic pH and enzymes being produced in the stomach. The formulation dosage form suffers premature release due to degradation of polymer in gastrointestinal pH [3] and it reduces targeted action of the encapsulated drug. Hence, more time/pH controlled DDS should be designed to overcome these obstacles
Cellulose and cellulose based derivatives are accepted as natural materials with good tolerance by the human body and are commonly used in medical and pharmaceutical applications such as targeted DDS [4,5]. The other important properties of cellulose polymers are biocompatibility with tissue and blood, non-toxicity and low cost [5].
HEC is an excellent derivative of cellulose with superior water retention and biocompatibility. It contains several –OH groups on its structure that allows it to be chemically modified by various means [6,7]. Recently, HEC-based swelling/floating gastroretentive DDS has been tried for its clinical relevance in healthy volunteers [8]. The HPC is another well-known polymer wherein few –OH group in repeating sugar units are hydroxypropylated using propylene oxide [9]. The high glass transition temperature of HPC confers great stability and restricts drug diffusion, recrystallization during storage. Moreover, the free –OH group of HEC readily form the hydrogen bond with a carbonyl group of pharmaceuticals, which provides stability in the solid state [10-12].
Nevertheless HEC and HPC matrices due to high hydrophilicity on few instances leads to a premature release of drugs and that need to be modulated in order to enhance its pharmaceutical applicability. Biofield is being generated by a human body that causes a paramount effect on surroundings. Mr. Mahendra Trivedi is well known to change the characteristics of various living and non-living things in controlled research experiments through his biofield, referred herein as Biofield treatment. The said Biofield has significantly changed the atomic, crystalline, and thermal characteristics of various materials such as metals, ceramics and carbon allotropes [13-20]. Recently it was reported that the use of biofield has significantly improved the yield and quality of various agricultural products [21-23]. Furthermore biofield has significantly optimized antibiotic sensitivity and produced biochemical reactions which further changed the characteristics of pathogenic microbes [24-26]. Additionally the effects of biofield on growth and anatomical characteristics of the herb Pogostemon cablin used in perfumes, in incense/insect repellents, were recently investigated [27].
In the present work, HEC and HPC polymers were treated with Biofield. The treated polymers were characterized by XRD, DSC, TGA and CHNSO analysis.
Materials And Methods
The Hydroxyethyl cellulose (HEC) and hydroxypropyl cellulose (HPC) were procured from Sigma Aldrich, USA. The HEC and HPC powders were treated with Mr. Trivedi’s biofield at different times, and samples were subjected to polymer characterization.
Polymer samples (HEC and HPC) from one batch was divided into three different parts. One was considered as a control while the remaining two were exposed to different amount of Mr. Trivedi’s biofield at different time intervals and named as T1 and T2 (treated samples). In order to avoid errors, only standardized parameters were used for comparison.
Characterization
CHNSO analysis: The control and treated polymers (HEC and HPC) were analyzed using CHNSO Analyzer Model Flash EA 1112 series, Thermo Finnigan, Italy.
X-ray diffraction (XRD) study: X-ray diffraction analysis of the polymer samples (HEC and HPC) were carried out using a power Phillips Holland PW 1710 X-ray diffractometer system. A copper anode with nickel filter was used. The wavelength of the radiation was 1.54056 Ǻ. The data were obtained in the form of 2θ versus intensity (a.u) chart. The crystallite size was calculated from XRD data using following formula.
Crystallite size=kλ/b Cos θ (1)
Where λ is the wavelength and k is the equipment constant with a value of 0.94.
Differential scanning calorimetry (DSC): DSC (HEC and HPC) were recorded with Pyris-6 DSC Perkin Elmer, at a heating rate of 10°C/ min with a nitrogen flow of 5 mL/min.
Thermogravimetric analysis (TGA): The thermal stability of the (HEC and HPC) was measured on a Mettler Toledo simultaneous TGA thermogravimetric analyzer (TGA) and differential thermal analysis (DTA). The samples were heated from room temperature to 400°C with a heating rate of 5°C/min under oxygen atmosphere
Results And Discussion
CHNSO analysis
CHNSO analysis was carried out to investigate the elemental composition in the treated HEC and HPC polymers. The CHNSO results are presented in Table 1. The control HEC polymer showed 43.62% carbon, 7.33% hydrogen, and 28.93% oxygen. The treated HEC showed 15.20% and 9.20% increased content of oxygen and hydrogen, respectively as compared to control. Similarly, the treated HPC polymer showed marked increase in percentage oxygen (7.09%) and hydrogen and (22.40%) as compared to control. Additionally the HEC showed a decrease in percentage nitrogen by -11.03 % but HPC did not show any change because it does not have nitrogen on its structure. Similarly the treated HEC polymer showed -0.19% decrease in percentage carbon as compared to control and HPC showed 0.05% increase in percentage carbon as compared with control polymer. This confirms that biofield treatment changed the elemental composition of HEC and HPC.
| Parameter | Hydroxyethyl cellulose | Hydroxypropyl cellulose |
|---|---|---|
| Nitrogen control | 0.39 | 0.00 |
| Nitrogen treated | 0.35 | 0.00 |
| % Change in nitrogen | -11.03 | - |
| Carbon control | 43.62 | 52.67 |
| Carbon treated | 43.53 | 52.70 |
| % Change in carbon | -0.19 | 0.05 |
| Hydrogen control | 7.33 | 9.02 |
| Hydrogen treated | 8.00 | 9.66 |
| % Change in hydrogen | 9.20 | 7.09 |
| Oxygen control | 28.93 | 23.92 |
| Oxygen treated | 33.33 | 29.28 |
| % Change in oxygen | 15.20 | 22.40 |
Table 1: CHNSO analysis of hydroxyethyl cellulose and hydroxypropyl cellulose.
X-ray diffraction
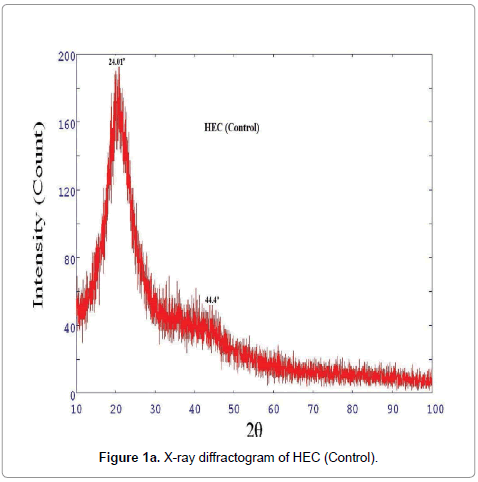
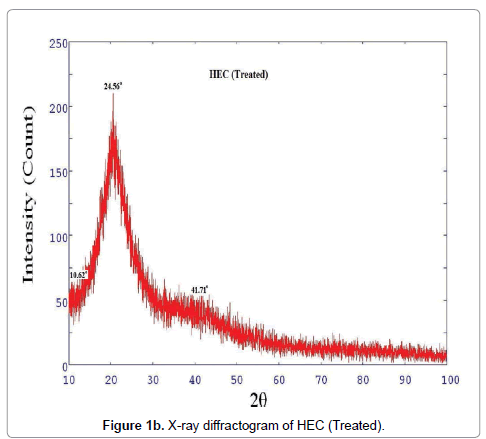
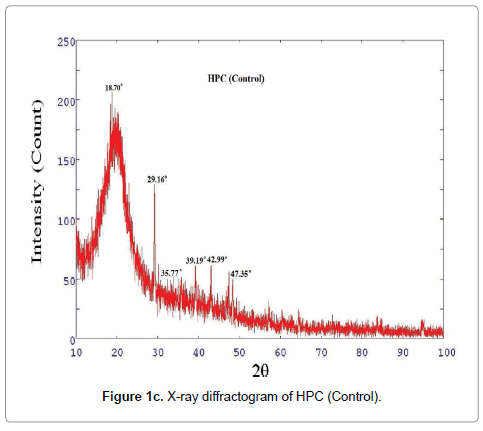
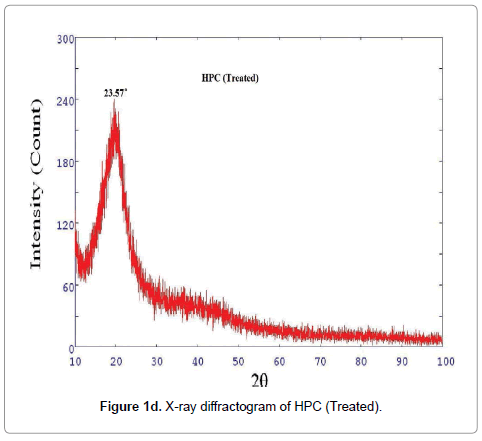
XRD diffractogram of control and biofield treated polymer HEC is illustrated in Figures 1a and 1b, respectively. The X-ray diffractogram of control HEC showed typical semi-crystalline nature of the polymer (Figure 1a). The XRD showed a peak at 2θ=24.01°. Another broad and diffused peak was observed at 2θ=44.4°. The treated HEC polymer (Figure 1b) showed similar semi-crystalline nature with a prominent peak at 2θ=24.56°. The XRD of HEC showed another peaks at 2θ=10.62° and 41.71°. The crystallite size was calculated from XRD diffractogram of HEC polymer using Scherrer’s formula (kλ/b Cos θ). The crystallite size of the control HEC polymer was 9.51 nm; however after treatment it was increased to 88.99 nm. It was observed that treated HEC showed 835% increase in crystallite size. This significant improvement in crystallite size might be due to the reason that biofield is directly acting on HEC molecules leading to expansion of crystals. Kim et al. observed similar results during their studies on thermal decomposition of native cellulose; they suggested that crystallite size increases due to increase in crystalline indices [28]. The corresponding increase in crystallite size was due to disappearance of amorphous regions in cellulose (HEC) reflecting improvement in crystallinity. The XRD diffractogram of HPC (control and treated) polymer is showed in Figures 1c and 1d, respectively, which confirmed coexistence of both amorphous and crystalline regions in the HPC polymer. The XRD diffractogram showed (Figure 1c) a broad peak at 2θ=18.70° and few crystalline peaks were observed at 2θ=29.16°, 35.77°, 39.19°, 42.99°, 47.35°. Nevertheless, the biofield treated HPC polymer showed (Figure 1d) a peak at 2θ=23.57°, which showed amorphous nature of the treated polymer.
Differential Scanning Calorimetry (DSC)
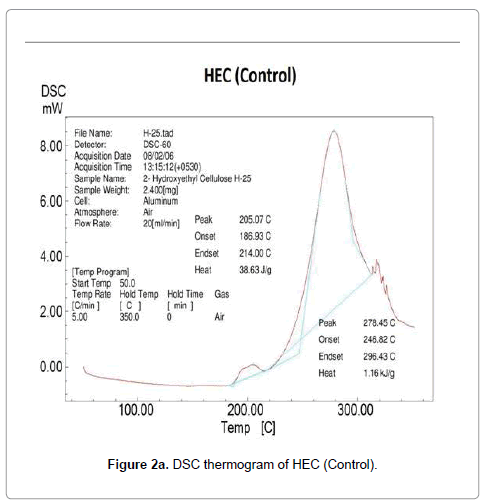
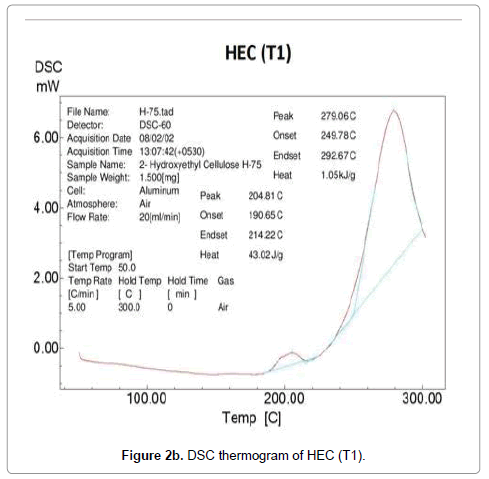
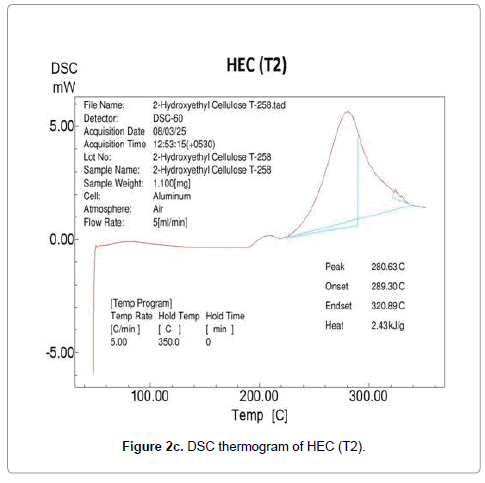
Differential scanning calorimetry was used as an excellent technique to measure the glass transition and melting nature of the polymer. DSC thermogram of HEC control and treated polymer is presented in Figure 2. The control HEC polymer showed an endothermic inflexion at 189°C due to a segmental motion of the polymer molecules reflecting the glass transition temperature of HEC (Figure 2a). In general the amorphous region present in a polymer shows the glass transition temperature. The DSC of HEC displayed a broad endothermic peak at 278°C confirming the melting temperature of the polymer. After biofield treatment, the DSC thermogram of HEC (T1 and T2) showed an elevation in both glass transition and melting temperature. The glass transition was increased to 192°C (T1) (Figure 2b) and 210°C (T2) (Figure 2c) in both the treated HEC samples as compared to control samples.
Based on this result, we hypothesize that the biofield is directly acting upon the molecules and restricting the segmental motion in the amorphous region thereby elevation in glass transition was observed. Paradkar et al. showed that high glass transition of polymer, promotes stability and restricts drug diffusion and recrystallization during storage; further the melt viscosity of the polymer make it suitable for hot melt extrusion processing [9]. This confirms that treated HEC (T1 and T2) polymer might be suitable for the DDS. The HEC polymer (T1 and T2) showed an increase in melting temperature (279°C and 280°C) reflecting improved thermal stability of HEC after biofield treatment.
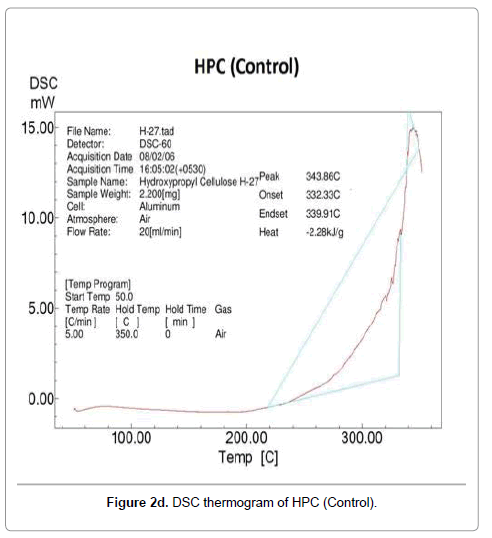
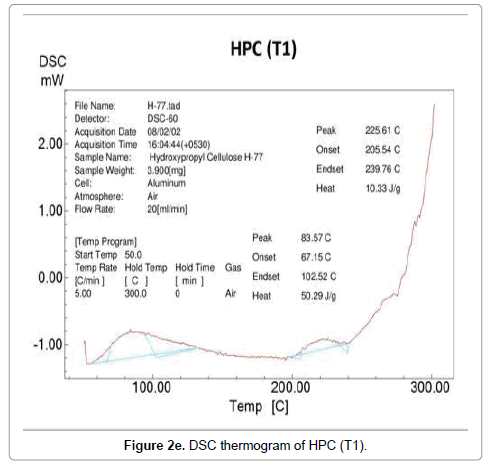
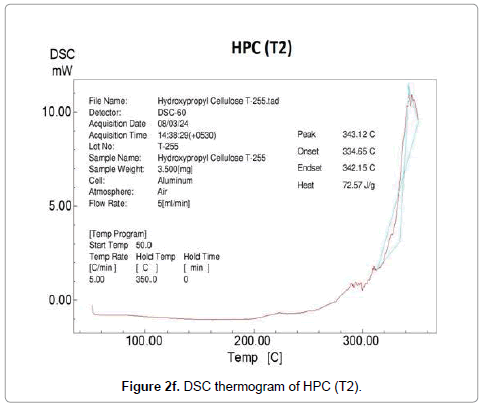
Contrarily no glass transition property was observed in DSC thermograms of control and treated HPC polymer. The DSC thermogram of control HPC showed (Figure 2d) an endothermic peak at 343°C that was responsible for its melting temperature. After biofield treatment, the thermograms of HPC (T1 and T2) showed (Figure 2e) decrease in melting temperature of T1 sample (225°C); however T2 showed (Figure 2f) similar melting peak (343°C) as showed by the control HPC. The high melting temperature of HPC (T2) indicated that a high amount of thermal energy was needed in order to disturb the long-range order of the crystals.
Thermogravimetric analysis (TGA)
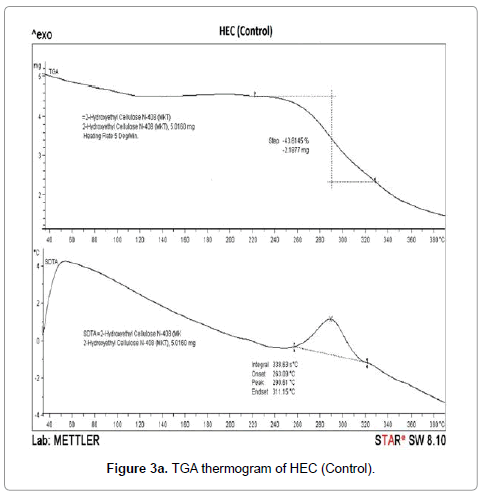
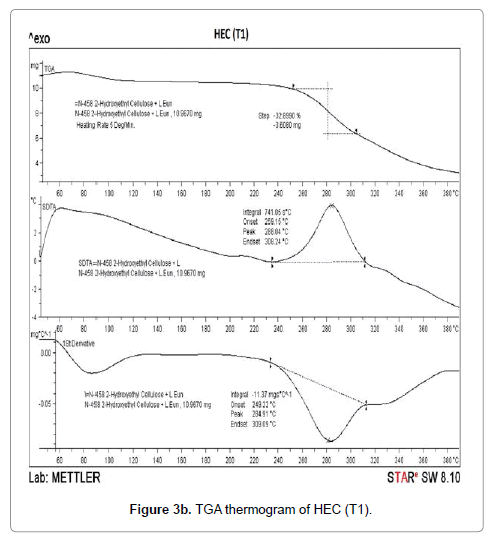
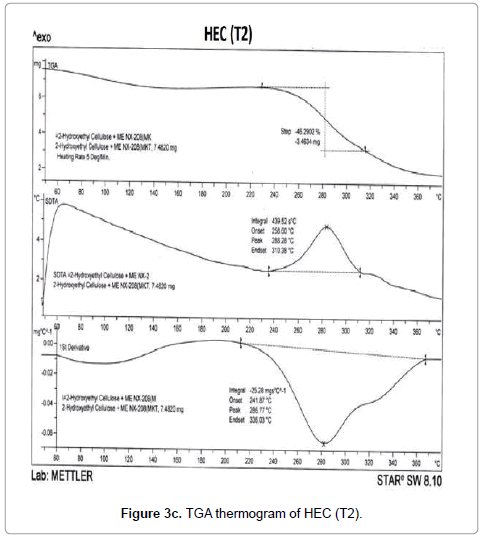
The thermogravimetric analysis is a technique to investigate the thermal stability of the polymers. TGA thermograms of HEC control polymer and treated sample are shown in Figures 3a-3d. TGA thermogram of control HEC polymer exhibited one step thermal degradation pattern (Figure 3a). Control HEC started to decompose at 240°C (initial decomposition temperature), and it stopped at 330°C.The HEC control polymer lost 43.61% of its original weight during this process. The treated HEC polymer (T1 and T2) displayed identical single step thermal degradation process. The polymer (T1) started to lose its weight at 252°C and ended at 305oC. The initial decomposition temperature (IDT) was increased in the treated HEC (T1) polymer (252°C) (Figure 3b) which showed its higher thermal stability. However the initial decomposition temperature was decreased in HEC (T2) sample (232°C) (Figure 3c).
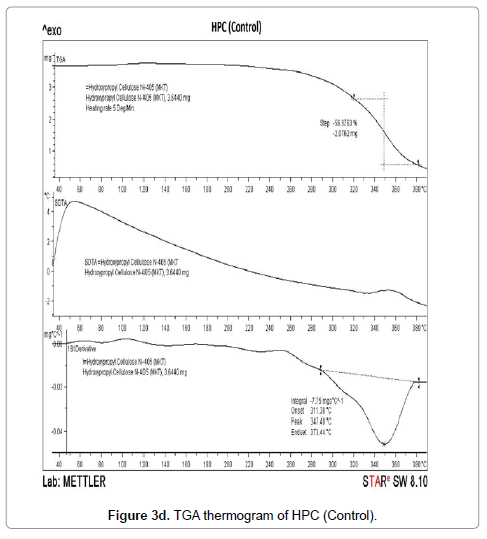
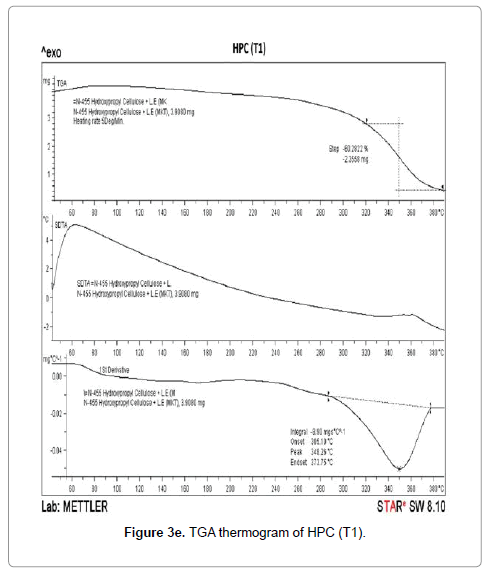
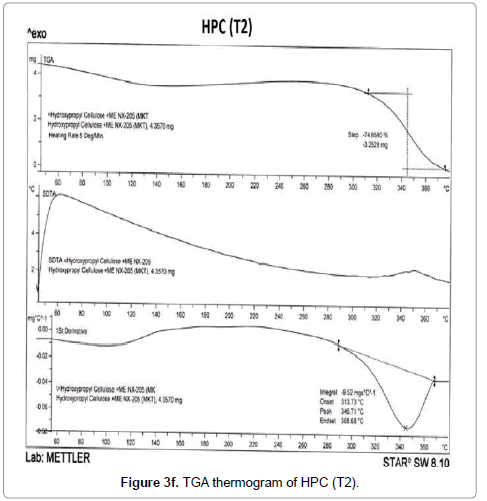
The TGA thermograms of HPC polymer (control) and treated HPC are illustrated in Figures 3d-3f. The control HPC polymer showed (Figure 3d) much higher initial decomposition temperature at 320°C and degradation terminated at 384°C. The polymer lost 56.97% of its original weight during this thermal process. The biofield treated HPC (T1) (Figure 3e) showed an improvement in initial decomposition temperature (322°C) which correlates well with its superior thermal stability. Though we observed a minimal decrease in initial decomposition temperature of T2 sample (315°C) as compared to control (Figure 3f). This result was well supported by our DSC observation of the HEC and HPC.
The CHNSO results confirmed significant increase in percentage oxygen and hydrogen of treated HEC and HPC polymers as compared to control samples. We presume that substantial increase in hydrogen and oxygen elements in the polymers (HEC and HPC) might have improved the hydrogen bonding. The strong hydrogen bonding may increase the crystallinity and thermal stability of the polymers which we have observed in treated HEC. Moreover the treated HEC had shown increased glass transition temperature as compared to control that might improve the drug stability in gastro retentive drug delivery and effectively reduce the premature drug diffusion from the matrix. Hence these results confirmed that treated polymers (HEC and HPC) could be an interesting candidate for oral targeted DDS. Furthermore, a few experiments are required to investigate the potential of biofield treated polymers (HEC and HPC) in DDS.
Conclusion
Mr. Trivedi’s biofield treatment had substantially improved the physicochemical properties of HEC and HPC polymers. XRD showed that treatment with biofield had significantly enhanced the crystallite size by 835% in treated HEC as compared to control and possibly this increased the crystallinity. It was presumed that enhanced crystalline indices in treated HEC caused increase in crystalline size. DSC showed the increase in melting temperature of treated HEC and HPC as compared to control polymers. It was postulated that biofield treatment probably assisted the formation of long range order in crystal of polymers (HEC and HPC) which increased the melting temperature and thermal stability. CHNSO results showed substantial increase in percentage hydrogen and oxygen which confirmed that biofield had possibly induced structural changes in the treated polymers (HEC and HPC). Thermal analysis by TGA showed significant improvement in thermal stability of treated HEC (T1) and HPC (T1) as compared to control. ‘We hypothesize that biofield treatment probably caused changes at structural and atomic level due to weak interactions in the polymers. Based on the results the treated polymers could be used as a matrix for oral targeted DDS
Acknowledgements
The authors would like to thank all the laboratory staff for their assistance during the various instrument characterization. We thank Dr. Cheng Dong of NLSC, Institute of Physics, and Chinese Academy of Sciences for permitting us to use Powder X software for analyzing XRD results.
References
- Hearnden V, Sankar V, Hull K, Juras DV, Greenberg M, et al. (2012) New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv Drug Deliv Rev 64: 16-28.
- Nicolazzo JA, Reed BL, Finnin BC (2005) Enhancing the buccal mucosal uptake and retention of triamcinolone acetonide. J Control Release 105: 240-248.
- Al-Hilal TA, Alam F, Byun Y (2013) Oral drug delivery systems using chemical conjugates or physical complexes. Adv Drug Deliv Rev 65: 845-864.
- Agarwal T, Narayana SN, Pal K, Pramanik K, Giri S, et al. (2015) Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int J Biol Macromol 75: 409-417.
- Liesiene J, Matulioniene J (2004) Application of water-soluble diethylaminoethylcellulose in oral drug delivery systems. Reactive Functional Polymers 59: 185-191.
- Wang W, Wang J, Kang Y, Wang A (2011) Synthesis, swelling and responsive properties of a new composite hydrogel based on hydroxyethyl cellulose and medicinal stone. Composites: Part B : Engineering 42: 809-818.
- Lin SB, Wu JH, Yao KD, Cai KY, Xiao CM, et al. (2004) Study of microstructure and properties of HEC-g-AA/SiO2 organic–inorganic hybrid materials. Composite Interface 11: 271-276.
- Chen RN, Ho HO, Yu CY, Sheu MT (2010) Development of swelling/floating gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. Euro J Pharma Sciences 39: 82-89.
- Paradkar A, Kelly A, Coates P, York P (2009) Shear and extensional rheology of hydroxypropyl cellulose melt using capillary rheometry. J Pharm Biomed Anal 49: 304-310.
- Sarode AL, Sandhu H, Shah N, Malick W, Zia H (2013) Hot melt extrusion for amorphous solid dispersions: temperature and moisture activated drug-polymer interactions for enhanced stability. Mol Pharm 10: 3665-3675.
- Sarode AL, Malekar SA, Cote C, Worthen DR (2014) Hydroxypropyl cellulose stabilizes amorphous solid dispersions of the poorly water soluble drug felodipine. Carbohydrate Polymers 112: 512-519.
- Warren DB, Benameur H, Porter CJ, Pouton CW (2010) Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: A mechanistic basis for utility. J Drug Target 18: 704-731.
- Trivedi MK, Tallapragada RR (2008) A transcendental to changing metal powder characteristics. Metal Powder Report 63: 22-28, 31.
- Dabhade VV, Tallapragada RR, Trivedi MK (2009) Effect of external energy on atomic, crystalline and powder characteristics of antimony and bismuth powders. Bull Mater Sci 32: 471-479.
- Trivedi MK, Tallapragada RR (2009) Effect of superconsciousness external energy on atomic, crystalline and powder characteristics of carbon allotrope powders. Mater. Res. Innovations 13: 473-480.
- Trivedi MK, Patil S, Tallapragada RM (2012) Thought Intervention through Biofield Changing Metal Powder Characteristics Experiments on Powder Characterisation at a PM Plant, Springer Berlin Heidelberg, Editor : Wei Deng, Lecture Notes in Electrical Engineering-Future Control and Automation 173: 247-252.
- Trivedi MK, Patil S, Tallapragada RM (2013) Effect of Biofield Treatment on the Physical and Thermal Characteristics of Vanadium Pentoxide Powders. J Material Sci Eng S11: 001.
- Trivedi MK, Patil S, Tallapragada RM (2013) Effect of bio field treatment on the physical and thermal characteristics of Silicon, Tin and Lead powders. J Material Sci Eng 2: 125.
- Trivedi MK, Patil S, Tallapragada RM (2014) Atomic, Crystalline and Powder Characteristics of Treated Zirconia and Silica Powders. J Material Sci Eng 3: 144.
- Trivedi MK, Patil S, Tallapragada RMR (2015) Effect of Biofield Treatment on the Physical and Thermal Characteristics of Aluminium Powders. Ind Eng Manage 4: 151.
- Shinde V, Sances F, Patil S, Spence A (2012) Impact of Biofield Treatment on Growth and Yield of Lettuce and Tomato. Australian Journal of Basic and Applied Sciences 6: 100-105.
- Sances F, Flora E, Patil S, Spence A, Shinde V (2013) Impact Of Biofield Treatment On Ginseng And Organic Blueberry Yield. Journal of Agricultural Science 35: 1991-8178.
- Lenssen AW (2013) Biofield and Fungicide Seed Treatment Influences on Soybean Productivity, Seed Quality and Weed Community. Agricultural Journal 8: 138-143.
- Trivedi M, Patil S (2008) Impact of an external energy on Staphylococcus epidermis (ATCC–13518) in relation to antibiotic susceptibility and biochemical reactions – An experimental study. Journal of Accord Integrative Medicine 4: 230-235.
- Trivedi M, Patil S (2008) Impact of an external energy on Yersinia enterocolitica (ATCC–23715) in relation to antibiotic susceptibility and biochemical reactions: An experimental study. The Internet Journal of Alternative Medicine 6: 2.
- Trivedi M, Bhardwaj Y, Patil S, Shettigar H, Bulbule A (2009) Impact of an external energy on Enterococcus faecalis (ATCC–51299) in relation to antibiotic susceptibility and biochemical reactions – An experimental study. Journal of Accord Integrative Medicine 5: 119-130.
- Patil SA, Nayak GB, Barve SS, Tembe RP, Khan RR (2012) Impact of Biofield Treatment on Growth and Anatomical Characteristics of Pogostemon cablin (Benth). Biotechnology 11: 154-162.
- Kim UJ, Eom SH, Wada M (2010) Thermal decomposition of native cellulose: Influence on crystallite size. Polymer Degradation Stability 95: 778-781.
Citation: Trivedi MK, Nayak G, Patil S, Tallapragada RM, Mishra R (2015) Influence of Biofield Treatment on Physicochemical Properties of Hydroxyethyl Cellulose and Hydroxypropyl Cellulose. J Mol Pharm Org Process Res 3: 126 Doi: 10.4172/2329-9053.1000126
Copyright: ©2015 Trivedi MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 21463
- [From(publication date): 8-2015 - Apr 19, 2024]
- Breakdown by view type
- HTML page views: 16805
- PDF downloads: 4658